Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Al-zheimer’s disease (Aarsland et al., 2021). PD prevalence rate and incidence are increasing because of the longer life expectancy and several other environmental factors (Lee et al., 2018). Its pathology mainly includes the death of dopaminergic neurons in the substantia nigra pars compacta (SNc) of brain, deposition of Lewy bodies i.e. aggregates of misfolded protein predominantly α-synuclein and dysfunctional mitochondria (Gómez-Benito et al., 2020). PD patients experience a variety of motor and non-motor symptoms that include tremors, rigidity, bradykinesia (slowness of movement), postural instability, olfactory dysfunction, gastrointestinal issues (e.g., constipation), mood disorders (e.g., depression and anxiety), cognitive impairment, sleep disturbances, and autonomic dysfunctions such as urinary problems and drooling (Sveinbjornsdottir, 2016). Most cases in PD are driven by environmental factors highlighting the need for analysis of environmental risk factors which can trigger and/or aggravate PD conditions.
Gut microbiota consisting of dynamic microbial community contains bacterial population as its main component. It establishes a symbiotic relationship with the host and its activities affect the metabolism, physiology and health status of the host. Adult gut microbiota is dominated by Bacteroidetes and Firmicutes phyla. Gut microbiota, in its balanced form, plays a crucial role in gut-brain axis (GBA) by neuronal, immune and endocrine pathways thereby effecting gastrointestinal barrier, immune response and neurological development. Gut dysbiosis, on the other hand, plays a critical role in the development and progression of various neurodegenerative disorders via GBA. Dysbiosis leads to changes in cognition, motor function and overall behavior by effecting the CNS through neural, endocrine and immune pathways (Zhang et al., 2022).
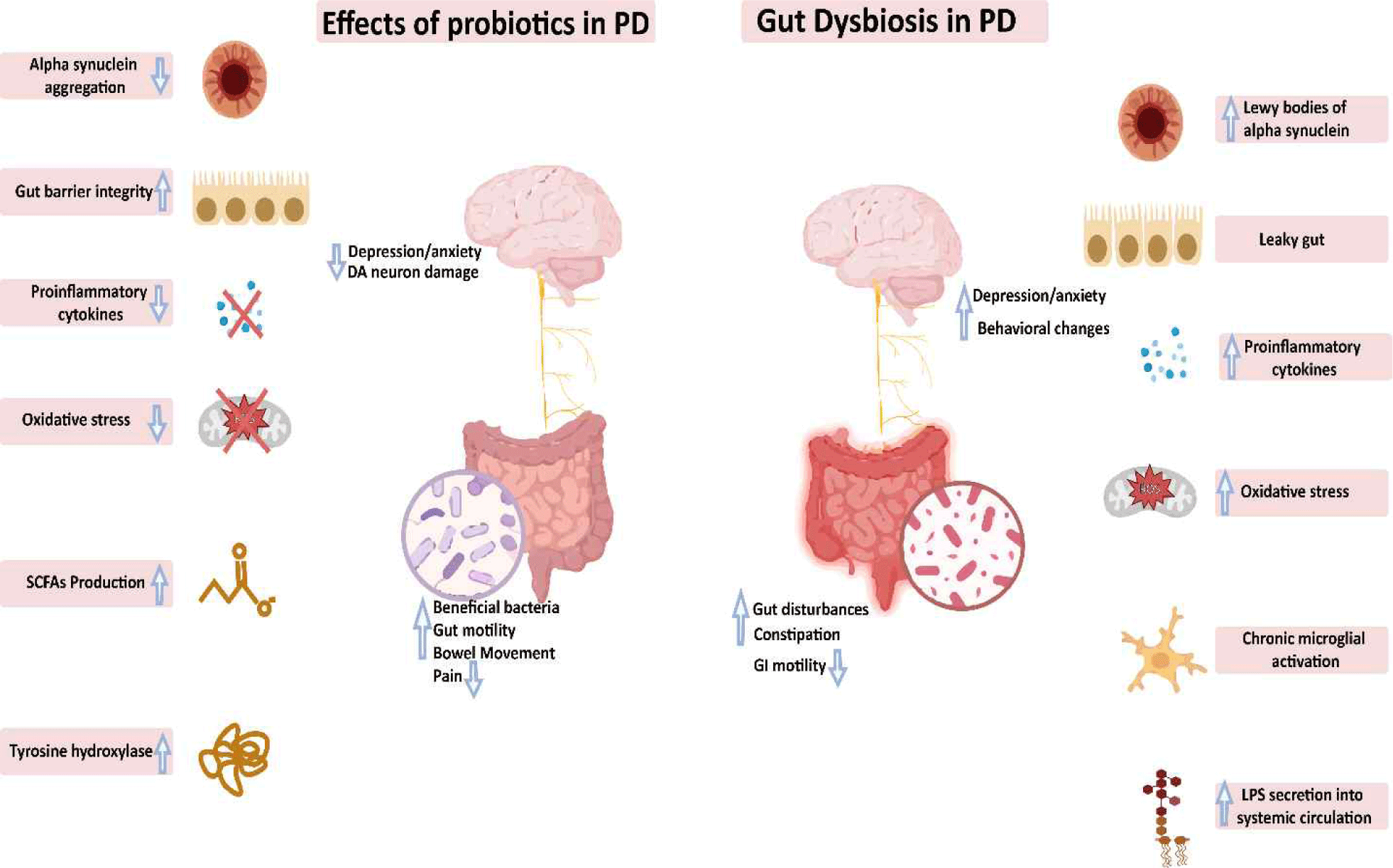
This review aims to provide updated information on the role of gut dysbiosis in PD trigger and progression and the potential of probiotics in ameliorating the various pathologies involved in PD complications. Fig. 1 provides a comparative overview of how probiotics modulate gut–brain interactions in PD relative to the pathological effects of gut dysbiosis.
Gut Microbiota and Gut-Brain Axis
Gut microbiota is an integral part of gut physiology. It plays role in metabolism of foods, nutrients, drugs and other metabolites. Due to the significant role of gut microbiota in host physiology, the concept of the microbiota-gut-brain axis was introduced to describe the bidirectional interaction between the gut microbiota, the gastrointestinal tract, and the central nervous system. The gut-brain axis is a bidirectional communication network that connects the central nervous system (CNS), and the enteric nervous system (ENS) also called the second brain (Rhee et al., 2009). It integrates emotional and cognitive centers of the brain with intestinal functions through neural, hormonal, immune, and metabolic pathways (Dinan & Cryan, 2017).
Gut microbiota plays a crucial role in brain development and cognitive function through its influence on neurotransmitter regulation, such as brain-derived neurotrophic factor (BDNF) and serotonin, and the stress-regulating hypothalamic pituitary-adrenal axis (Morais et al., 2021). Gut microbiota has an important role in GBA since it not only interacts with the ENS and intestinal cells but also with the CNS. Bacterial colonization has central role in the development of both, the CNS and ENS. Studies on germ-free animals reveal that gut microbiota is essential for the development and maturation of both the enteric and central nervous systems, influencing neurotransmitter expression, gut motility, and sensory-motor functions (Morais et al., 2021). Studies on disorders like Inflammatory bowel disease (IBD) and autism confirm their potential role in amelioration of both kind of disorders i.e. the intestinal and depressive-like disorders (Kang et al., 2019; Khan et al., 2019).
Gut Dysbiosis and PD
Recent research has highlighted the gut-brain connection and the role of dysbiosis in gut microbial community in PD, with the role of gut microbiome in disease onset becoming more and more clear. Gut microbiome dysbiosis can result from age, poor quality of diet, or some disease conditions and can in turn lead to lack of essential nutrients, increased toxins which can cause inflammation and neurodegeneration (Kwon et al., 2024). PD patients exhibit significant alterations in gut microbiota composition, including reduced levels of beneficial butyrate-producing bacteria and increased pro-inflammatory taxa. Dysbiosis compromises intestinal barrier integrity, increasing permeability to lipopolysaccharides (LPS), which can trigger systemic and neuroinflammation. Metagenomic analysis reveals that the genes involved in metabolism are down regulated, while those involved in LPS biosynthesis and type III secretion system (a complex needle-like protein structure used by Gram-negative bacteria o directly inject their effector protein into the host cells to promote infection) are upregulated which can lead to increased gut inflammation, chronic immune activation and eventually neurodegeneration. This inflammation promotes oxidative stress and α-synuclein aggregation in the enteric nervous system, possibly initiating a prion-like spread of pathology to the brain (Jain et al., 2023; Keshavarzian et al., 2015; Scheperjans et al., 2015). These findings suggest that gut microbiota imbalances may not only exacerbate PD progression but could also serve as potential biomarkers or targets for early therapeutic interventions. Table 1 enlists observed variation in bacterial groups and their possible association with PD condition analyzed through pyrosequencing, quantitative RT-PCR, high-throughput amplicon sequencing reported by several studies (Hasegawa et al., 2015; Keshavarzian et al., 2015; Sampson et al., 2016; Scheperjans et al., 2015).
Braak et al. presented ‘ascending anatomical theory’ suggesting the trigger of PD from gut and its gradual shifting towards the brain (Braak et al., 2003). This is supported by the observations that suggest that i) motor symptoms of PD are preceded by gastrointestinal symptoms (Abbott et al., 2001); ii) IBD can increase PD’s increased incidence (Park et al., 2019); iii) pathological changes in PD may be preceded by ENS changes (Stokholm et al., 2016); iv) PD involves reduced levels of tight junction proteins and leaky gut (Clairembault et al., 2015) v)α-Syn aggregation originates in the gut and then is transported to CNS (Klingelhoefer & Reichmann, 2015); vi) FMT can improve PD like conditions in animal models as well as PD patients (Sampson et al., 2016; Shekar et al., 2025).
Contradictory results reported by some other studies make the gut origin of PD questionable. For example, α-syn transmission has been said to be bidirectional in gut-brain axis. Additionally, autopsy data also doesn’t confirm this hypothesis. Thus, gut dysbiosis may play an important role in disease initiation or progression in some patients, but evidence of variable pathology and asymptomatic individuals with advanced α-synuclein burden highlights clear heterogeneity in PD. Consequently, GBA dysfunction is best viewed as one of several interacting pathogenic pathways, rather than a singular causal route. Whether PD pathology originates from gut is still debatable, however, involvement of gut health in this regard is undeniable (Zhang et al., 2023).
Probiotics as a Therapeutic Strategy for PD Amelioration
Probiotics are defined as the “live microorganisms which when administered in adequate amounts confer a health benefit on the host”. While their general health benefits are known for a long time, advancements in the field of high-throughput sequencing and omics have made it possible to explore their potential in the amelioration of specific disease conditions (Hasnain et al., 2024).
Probiotics offer promising potential for managing PD by addressing gastrointestinal dysfunction, neuroinflammation, oxidative stress, immune dysregulation, and modulation of α-synuclein aggregation which are intricately linked to the disease’s progression. Evidence suggests probiotics can alleviate PD-associated constipation, enhance gut motility, and improve gastric emptying, thereby optimizing medication absorption. Probiotics like Lactobacillus rhamnosus and Bifidobacterium longum have been shown to modulate brain neurochemistry, reduce anxiety, and improve stress responses (Castelli et al., 2021; Gazerani, 2019; Wang et al., 2019). Emerging research also highlights their potential to influence motor and cognitive symptoms, although the precise mechanisms and long-term effects require further investigation. The key mechanisms through which probiotics ameliorate Parkinson’s disease–related health outcomes are summarized below.
Gut Health Improvement
Probiotics improve gut health by restoring microbial balance through competitive exclusion of pathogens, biofilm formation, and cross-feeding interactions that stabilize the gut microbiota (Hill et al., 2014; Salas-Jara et al., 2016). They strengthen intestinal barrier integrity by upregulating tight junction proteins and mucus secretion while reducing gut inflammation via suppression of pro-inflammatory cytokines. Probiotic-driven increases in short-chain fatty acids, particularly butyrate, further support epithelial health and anti-inflammatory signaling (Klaenhammer et al., 2012; Sanders et al., 2019; Toscano et al., 2017). Clinically, these effects translate into improved gastrointestinal motility and significant relief of constipation in patients with PD (Cassani et al., 2011).
Modulation of Immunity and Inflammation
Probiotics modulate both innate and adaptive immune responses by enhancing phagocytosis and antibody production while suppressing pro-inflammatory cytokines and promoting anti-inflammatory mediators, thereby reducing intestinal and systemic inflammation (Klaenhammer et al., 2012; Sanders et al., 2019). in vitro and animal studies further show reduced neuroinflammation, inhibition of inflammatory signaling, and activation of anti-inflammatory regulators such as proliferator-activated receptor gamma (PPAR-γ), contributing to dopaminergic neuroprotection (Castelli et al., 2020; Magistrelli et al., 2019).
Neuronal Protection and α-Synuclein Modulation
Multiple preclinical studies demonstrate that probiotics preserve nigral dopaminergic neurons, improve motor function, and increase tyrosine hydroxylase–positive neurons in genetic and toxin-induced PD models (Castelli et al., 2020; Hsieh et al., 2020). In addition, probiotic supplementation has been shown to reduce α-synuclein accumulation, potentially through modulation of host lipid metabolism, including sphingolipids and ceramides, which are implicated in PD pathogenesis (Goya et al., 2020; Huebecker et al., 2019).
Microbial and Microbial Metabolite–Driven Mechanisms
Probiotics alter gut microbial composition through competition, antagonism, biofilm formation, and cross-feeding interactions, promoting colonization resistance and microbial stability (Hill et al., 2014). These interactions enhance the production of short-chain fatty acids (SCFAs), particularly butyrate, which supports gut barrier integrity, reduces inflammation, and exerts neuroprotective effects (Sanders et al., 2019; Srivastav et al., 2019). Probiotics also produce neuroactive compounds and precursors, including gamma-aminobutyric acid (GABA), serotonin, dopamine, and acetylcholine, influencing gut-brain signaling (Kim et al., 2018). Additionally, probiotics strengthen gut barrier function by upregulating tight junction proteins and mucus secretion, limiting endotoxin translocation (Sanders et al., 2019; Toscano et al., 2017).The table below (Table 2) enlists effects of various probiotic strains and the mechanisms through which these improve PD conditions.
| Probiotic strain | Study model | Key findings | Reference |
|---|---|---|---|
| Lactobacillus casei Shirota | Patient study (RCT) | Improved bowel movements and reduced gastrointestinal symptoms in PD patients | (Cassani et al., 2011) |
| Lactobacillus acidophilus & Bifidobacterium infantis | Patient study (RCT) | Alleviated abdominal pain and bloating | (Georgescu et al., 2016) |
| Probiotic mix (L. acidophilus, B. bifidum, L. reuteri, L. fermentum) | Patient study (RCT) | Reduced MDS-UPDRS scores (Movement disorders society-unified parkinson’s disease rating scale) | (Tamtaji et al., 2019) |
| Bifidobacterium bifidum, Bifidobacterium longum, Lactobacillus rhamnosus, L. plantarum, Lactococcus lactis subsp. lactis | MitoPark PD mice | Improved motor function and reduced nigral dopaminergic neuronal degeneration in transgenic PD mice | (Hsieh et al., 2020) |
| Lactobacillus rhamnosus GG, Bifidobacterium animalislactis, Lactobacillus acidophilus | Mouse model | Increased butyrate production and neuroprotection in MPTP and rotenone models. | (Srivastav et al., 2019) |
| SLAB51 (multi-strain mix) | In vitro + Mouse model | Neuroprotection through anti-inflammatory and antioxidant pathways and behavioral improvement | (Castelli et al., 2020) |
| Lactococcus lactis subsp. cremori (engineered strain with GLP-1) | Mouse model | Improved dopaminergic neurons and reduced locomotor impairment; boosted beneficial gut microbiota, reduced proinflammatory molecules | (Fang et al., 2019) |
| Bacillus subtilis PXN21 | C. elegans Model | Reduced α-synuclein aggregation and enhanced clearance of aggregates via alterations in sphingolipid metabolism | (Goya et al., 2020) |
| Lactobacillus spp. and Bifidobacterium spp. | In vitro (Blood cells from PD patients) | Modulation of inflammatory cytokines and inhibition of pathogenic bacteria growth | (Magistrelli et al., 2019) |
| Multi-strain mix and prebiotic fibers | Patient study (RCT) | Enhanced stool consistency, frequency, and quality of life in PD-related constipation. | (Barichella et al., 2016) |
| Probiotics mixture | Patient study (RCT) | Reduced overall disease severity related to constipation in PD patients | (Tan et al., 2021) |
| B. longum 1714, B. breve 1205 | Mouse model | Enhanced spatial and non-spatial memory, reduced visceral pain | (Savignac et al., 2015) |
| Lactobacillus acidophilus, L. reuteri, Bifidobacterium bifidum, L. fermentum | Male Wistar rats | Improved rotational behavior, cognitive function and neuronal damage | (Alipour Nosrani et al., 2021) |
| Bifidobacterium breve A1 | Mouse model | Restored hippocampal synaptic plasticity, reversed CA1 spine density decline, and improved contextual fear extinction | (Ishii et al., 2021) |
| Streptococcus thermophilus CRL 808, L. plantarum CRL 2130, S. thermophilus CRL 807 | Mouse model | Increased TH-positive cell counts, reduced inflammatory cytokines, and elevated anti-inflammatory IL-10 in brain | (Perez Visñuk et al., 2020) |
| Clostridium butyricum | Mouse model | Ameliorated synaptic dysfunction, improved dopaminergic neuron loss, motor function and reversed gut microbiota dysbiosis | (Sun et al., 2021) |
| Lacticaseibacillus rhamnosus HA-114 | Mouse model | Improved hippocampal-dependent cognition deficits | (Xie & Prasad, 2020) |
Current Gaps and Future Directions
Role of gut dysbiosis is well reported in PD patients, and research both in vitro and in vivo describes a promising potential of probiotics for ameliorating PD related health outcomes. While Lactobacilli and Bifidobacteria, are well known for their probiotic potential, their abundance has been reported to be negatively correlated with PD status in recent research (Wallen et al., 2020; Zhang et al., 2023). The increased abundance of typically probiotic genera such as Lactobacillus and Bifidobacterium in PD should be carefully interpreted as these shifts likely reflect secondary ecological responses to intestinal inflammation, loss of SCFA-producing taxa, altered gut physiology, and PD-related medication use, underscoring dysbiosis rather than causality.
Similarly, SCFAs have been proposed to improve the PD condition but the same were inversely correlated with PD status in a mouse model (Sampson et al., 2016). These observations demand further in-depth analysis of large samples to confirm their role. Furthermore, standardized protocols for probiotics’ strain selection, dosage, and delivery methods are needed for their clinical application. Long-term safety and efficacy studies are challenging due to the progressive nature of PD and the reliance on clinical rating scales instead of reliable biomarkers.
Concerns such as the risk of small intestinal bacterial overgrowth (SIBO) in PD patients and interactions with levodopa metabolism warrant further investigation (Tan et al., 2021). Future research should focus on high-resolution investigations of gut microbiome and metabolomic alterations in PD, alongside personalized probiotic therapies suitable for individual microbiota profiles. The integration of multiomics approaches is expected to advance precision medicine, enabling targeted probiotic interventions that address specific pathological features of PD.
