Introduction
Obesity is now widely recognized as a major global health challenge, affecting more than 650 million adults worldwide and substantially increasing the risk of chronic metabolic diseases. This metabolic crisis acts as a precursor to type 2 diabetes, cardiovascular disease, and malignancies, driven largely by a chronic imbalance between energy intake and expenditure. Despite decades of research and public health interventions, conventional approaches including caloric restriction and increased physical activity have shown limited long-term efficacy, with relapse rates exceeding 80% within five years. Pharmacological interventions, while moderately effective, are often associated with adverse effects and high costs. Consequently, identifying biological drivers of overeating that can be targeted therapeutically is an urgent priority.
A breakthrough came with the discovery that trillions of microorganisms inhabiting our gastrointestinal tract—collectively known as the gut microbiota—play critical regulatory roles in host metabolism and feeding behavior. Seminal studies demonstrating that germ-free mice are protected from diet-induced obesity (Bäckhed et al., 2004) and that microbiota transfer from obese donors can confer obesity phenotypes in recipient mice (Ridaura et al., 2013; Turnbaugh et al., 2006) established the causal role of gut microbiota in metabolic regulation. The subsequent discovery that gut microbiota communicates bidirectionally with the central nervous system through what we now call the microbiota-gut-brain axis has fundamentally reshaped our understanding of feeding behavior (Mayer et al., 2015).
The gut-brain axis is best understood as an intricate bidirectional communication network connecting the gastrointestinal tract with the central nervous system. This concept has evolved considerably from the classical view of gut-brain communication, which focused primarily on neural and endocrine signaling. The contemporary framework—the microbiota-gut-brain axis—recognizes gut microbiota as active participants, not passive bystanders, in this dialogue (Mayer et al., 2022). The microbiota influences brain function and behavior through multiple mechanisms, including production of neuroactive metabolites, modulation of immune responses, regulation of gut barrier integrity, and direct interaction with enteric and central nervous systems.
In the context of feeding behavior, the microbiota-gut-brain axis integrates peripheral metabolic signals with central neural circuits controlling feeding behavior—defined in this review as the integrated processes of appetite initiation and termination, satiety signaling, meal size/frequency determination, food reward valuation, and energy intake homeostasis—as well as food reward and energy homeostasis (Fetissov, 2017). This integration occurs at multiple levels, from the gut lumen where bacteria ferment dietary substrates, through the intestinal epithelium where nutrient sensors and hormone-producing cells reside, to the vagal afferent neurons that transmit satiety signals to brainstem nuclei, and ultimately to hypothalamic and limbic regions that govern feeding decisions. Dysregulation of this axis, as observed in obesity and metabolic syndrome, disrupts the delicate balance between hunger and satiety signals, leading to hyperphagia, altered food preferences, and metabolic dysfunction (Torres-Fuentes et al., 2017).
This review synthesizes current understanding of the mechanisms by which the gut microbiota modulates feeding behavior and evaluates nutritional interventions targeting this axis. In this review, “feeding behavior” is defined broadly to encompass the physiological regulation of appetite (hunger and satiety), behavioral patterns including meal size, frequency, and regularity, as well as food preference and reward-driven eating. Regarding outcomes, we prioritize mechanistic evidence linking microbial signals (e.g., SCFAs, gut hormones) to central neural regulation, alongside clinical and physiological endpoints such as energy intake, body weight composition, and metabolic health markers. We focus on four primary communication pathways: neural signaling with emphasis on the vagal afferent pathway, endocrine signaling through gut hormones, metabolic signaling via short-chain fatty acids, and immune-mediated pathways. We then examine how dysbiosis contributes to feeding behavior disorders, particularly obesity, and critically evaluate dietary interventions including prebiotics, probiotics, and postbiotics. Additionally, we discuss feeding pattern manipulation as an emerging strategy and highlight advanced technologies that promise to accelerate translation from mechanistic insights to clinical applications. Our goal is to provide a focused, mechanistically-oriented review that bridges basic science discoveries with therapeutic opportunities in the burgeoning field of microbiome-based nutrition. Although multiple signaling routes contribute to gut microbiota–brain communication, this review focuses on four core pathways—neural, endocrine, metabolic, and immune signaling—because they represent the most consistently supported and integrative mechanisms linking gut microbiota to feeding behavior regulation. Other pathways mentioned in the text, such as bile acid signaling or microbial modulation of neurotransmitter synthesis, are discussed as complementary mechanisms where relevant, rather than as primary organizing frameworks of the review.
Communication Pathways: How Microbiota Regulate Feeding
Multiple communication pathways link the gut microbiota to central feeding circuits, and these often work synergistically to influence feeding behavior. An integrated overview of gut microbiota–brain communication pathways involved in feeding behavior regulation is illustrated in Fig. 1.
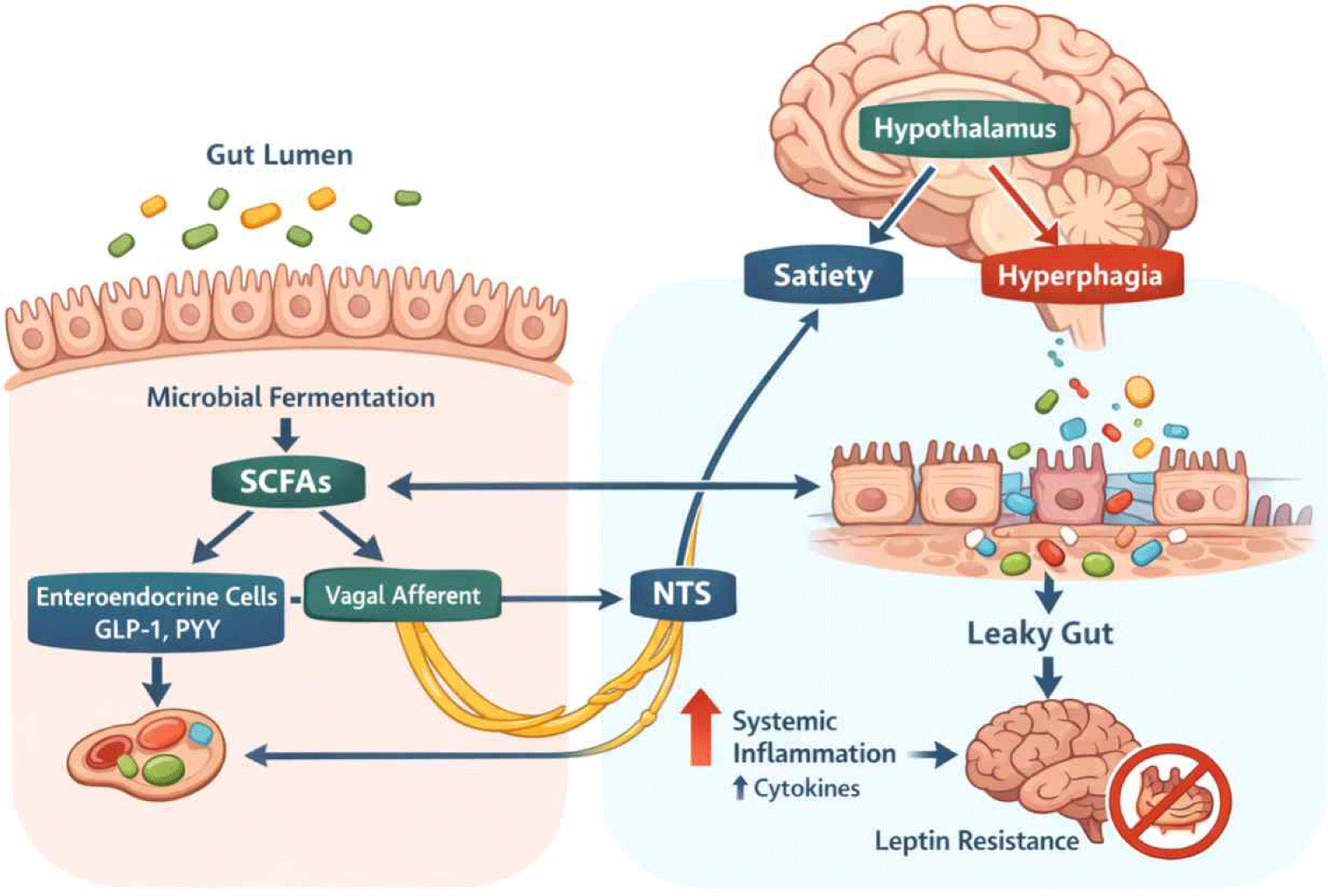
The vagus nerve serves as the primary neural conduit linking the gut and brain, with approximately 80% of vagal fibers being afferent, transmitting information from the periphery to the central nervous system. Vagal afferent neurons, whose cell bodies reside in the nodose ganglia, possess terminals in the intestinal mucosa where they detect mechanical stretch, nutrients, and microbial metabolites (Bauer et al., 2016). These signals are transmitted to the nucleus tractus solitarius (NTS) in the brainstem, which projects to hypothalamic nuclei including the arcuate nucleus and paraventricular nucleus, ultimately influencing feeding centers.
The gut microbiota modulates vagal signaling through several complementary mechanisms. Bacterial metabolites, particularly short-chain fatty acids produced from dietary fiber fermentation, can directly activate vagal afferents expressing G-protein coupled receptors such as GPR41 and GPR43 (Tolhurst et al., 2012). Second, microbiota-derived signals stimulate enteroendocrine cells to release hormones like cholecystokinin (CCK) and GLP-1, which activate vagal mechanoreceptors and chemoreceptors (Bellono et al., 2017). Third, the microbiota influences the structural and functional integrity of vagal neurons themselves.
High-fat diet-induced obesity profoundly disrupts the vagal afferent pathway’s structure and function. Studies have shown that gut microbiota composition modulates inflammation and structure of the vagal afferent pathway (De Lartigue, 2016; De Lartigue et al., 2011), with dysbiosis promoting inflammatory cytokine production that impairs vagal signaling. The good news is that interventions restoring beneficial microbiota through prebiotic supplementation can improve gut barrier function and enhance vagal pathway signaling. For example, dietary fiber supplementation increases SCFA production, which can reduce inflammation in nodose ganglia and restore vagal sensitivity to satiety signals (Li et al., 2018). These findings suggest that vagal dysfunction in obesity is at least partially reversible through microbiota-targeted interventions, representing a promising therapeutic avenue.
Notably, vagal signaling exhibits a high degree of functional specificity, as distinct populations of vagal afferent neurons respond preferentially to different nutritional and microbial stimuli. For instance, certain vagal neurons are selectively activated by dietary lipids, while others respond preferentially to proteins or carbohydrates. This specificity allows the brain to integrate detailed information about meal composition, contributing to nutrient-specific satiety and food choice. Understanding how the microbiota modulates these specialized vagal populations remains an active area of investigation.
The gastrointestinal tract is actually the body’s largest endocrine organ, producing numerous hormones that regulate feeding behavior and metabolism. Enteroendocrine cells (EECs), specialized epithelial cells scattered throughout the intestinal mucosa, sense luminal contents and release hormones in response to nutrients and microbial metabolites (Martin et al., 2019). Two major anorexigenic (appetite-suppressing) hormones, GLP-1 and PYY, are particularly relevant to microbiota-gut-brain signaling.
GLP-1, secreted primarily by L-cells in the distal small intestine and colon, promotes satiety through dual mechanisms. Peripherally, it slows gastric emptying and inhibits gastric acid secretion, prolonging the sensation of fullness. Centrally, GLP-1 accesses hypothalamic feeding circuits either by crossing the blood-brain barrier in regions with incomplete barrier function or by activating vagal afferents that project to the NTS and subsequently to hypothalamic nuclei (Rastelli et al., 2019). The gut microbiota profoundly influences GLP-1 secretion, with studies demonstrating that specific bacterial species and their metabolites can stimulate L-cells. For example, certain Lactobacillus strains increase GLP-1 production, while the beneficial bacterium Akkermansia muciniphila produces a protein (P9) that directly interacts with intestinal cells to enhance GLP-1 secretion (Everard et al., 2013).
Short-chain fatty acids represent a primary mechanism by which the microbiota stimulates GLP-1 and PYY release. These microbial metabolites activate free fatty acid receptors (FFAR2/GPR43 and FFAR3/GPR41) on L-cells, triggering hormone secretion (Psichas et al., 2015; Tolhurst et al., 2012). Propionate, in particular, has been shown to be a potent stimulator of GLP-1 and PYY release. Human studies have demonstrated that dietary supplementation with inulin-propionate ester, which delivers propionate to the colon, reduces energy intake and prevents weight gain in overweight adults (Chambers et al., 2015), effects associated with increased circulating PYY levels.
Conversely, the orexigenic (appetite-stimulating) hormone ghrelin, produced primarily by gastric P-cells, is also modulated by the gut microbiota. Germ-free mice exhibit altered ghrelin signaling compared to conventionally raised mice, and specific bacterial metabolites can influence gastric ghrelin production. However, the relationship between microbiota and ghrelin is complex and incompletely understood, with both positive and negative associations reported depending on the bacterial species and metabolic context.
Short-chain fatty acids—primarily acetate, propionate, and butyrate—are produced when gut bacteria ferment non-digestible carbohydrates and represent one of the most important mechanisms by which the microbiota influences host metabolism and feeding behavior (Canfora et al., 2015; Morrison and Preston, 2016). These three- to six-carbon fatty acids are produced at millimolar concentrations in the colon, with acetate being the most abundant, followed by propionate and butyrate in roughly 60:20:20 proportions, though these ratios vary with diet and microbiota composition.
SCFAs exert their effects through multiple complementary mechanisms. First, they serve as energy substrates, particularly for colonocytes (butyrate), hepatocytes (propionate), and peripheral tissues (acetate). Butyrate provides approximately 70% of the energy requirements of colonocytes and is crucial for maintaining intestinal epithelial barrier function. Second, SCFAs act as signaling molecules by activating G-protein coupled receptors, particularly GPR41 (FFAR3) and GPR43 (FFAR2), which are expressed in various tissues including enteroendocrine cells, adipocytes, and immune cells (Kimura et al., 2013; Samuel et al., 2008). Third, SCFAs function as histone deacetylase (HDAC) inhibitors, particularly butyrate, thereby influencing gene expression through epigenetic mechanisms.
For feeding behavior specifically, SCFAs promote satiety through multiple pathways. Activation of GPR43 on intestinal L-cells stimulates PYY and GLP-1 secretion, as described above (Tolhurst et al., 2012). Additionally, SCFAs can directly access the central nervous system and influence hypothalamic neurons controlling energy balance. Studies have shown that intracerebroventricular administration of acetate reduces food intake and activates pro-opiomelanocortin (POMC) neurons in the arcuate nucleus (Frost et al., 2014), which are critical mediators of satiety. Butyrate has been shown to reduce appetite and activate brown adipose tissue via the gut-brain neural circuit (Li et al., 2018).
The effects of SCFA supplementation on metabolic health and feeding behavior are complex and context-dependent. Studies with butyrate supplementation have revealed that metabolic responses are highly variable and associated with changes in gut microbiota composition and function (Lee et al., 2020). In some experimental cohorts, butyrate supplementation improved glucose tolerance and reduced adiposity (Gao et al., 2009), while in others, effects were minimal or absent. This heterogeneity underscores the importance of considering individual microbiota composition when designing SCFA-based interventions and suggests that baseline microbiota may determine responsiveness to such treatments (Sanna et al., 2019).
Importantly, different SCFAs may have distinct effects on appetite and metabolism. Propionate appears particularly effective at reducing energy intake in humans (Chambers et al., 2015), while butyrate shows more pronounced effects on insulin sensitivity and metabolic inflammation. Acetate, despite being the most abundant SCFA, may have paradoxical effects, with some studies suggesting it could stimulate rather than suppress appetite under certain conditions. Understanding these nuances is critical for developing effective SCFA-based therapeutic strategies.
Chronic low-grade inflammation, often termed “metaflammation” (metabolic inflammation), is a hallmark of obesity and metabolic syndrome. The gut microbiota plays a central role in this inflammatory state through effects on intestinal barrier integrity and systemic immune activation (Saad et al., 2016).
Under healthy conditions, the intestinal epithelium maintains a selective barrier that allows nutrient absorption while preventing translocation of bacteria and their products into the circulation. This barrier function depends on tight junction proteins (occludin, claudins, zona occludens-1) that seal the paracellular space between epithelial cells, and on the mucus layer produced by goblet cells that provides a physical separation between microbiota and epithelium. Dysbiosis, particularly the reduction in beneficial bacteria like Akkermansia muciniphila and increases in pro-inflammatory species, compromises barrier integrity, leading to increased intestinal permeability or “leaky gut” (Chakaroun et al., 2020).
Increased intestinal permeability allows translocation of bacterial components, particularly lipopolysaccharide (LPS), into the circulation. This metabolic endotoxemia activates innate immune receptors, especially Toll-like receptor 4 (TLR4), on immune cells and metabolic tissues, triggering production of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) (Cani et al., 2007). These cytokines have direct and indirect effects on feeding centers in the brain.
Inflammatory cytokines can access the central nervous system through multiple routes: active transport across the blood-brain barrier, transmission through circumventricular organs lacking a complete blood-brain barrier, and activation of vagal afferents that relay inflammatory signals to the brainstem. Once in the brain, cytokines activate microglia, the resident immune cells, which produce additional inflammatory mediators that can disrupt hypothalamic feeding circuits. Chronic hypothalamic inflammation has been shown to impair leptin and insulin signaling in neurons regulating energy balance, contributing to leptin resistance and sustained hyperphagia despite elevated adiposity (Schéle et al., 2013).
Interventions that improve gut barrier function and reduce metabolic endotoxemia can ameliorate these inflammatory effects. For example, supplementation with beneficial bacteria or their metabolites has been shown to increase tight junction protein expression, reduce circulating LPS levels, decrease pro-inflammatory cytokine production, and improve hypothalamic leptin sensitivity. These effects collectively contribute to improved appetite regulation and metabolic health.
The major gut microbiota–brain axis pathways regulating feeding behavior are summarized in Table 1, highlighting the integration of neural, endocrine, metabolic, and immune signaling.
Dysbiosis and Feeding Behavior Alterations
Disruption of the normal gut microbiota composition, termed dysbiosis, is associated with various feeding behavior disorders and metabolic diseases. Understanding these associations provides insight into potential therapeutic targets.
Obesity is characterized by consistent alterations in gut microbiota composition, most notably an increased Firmicutes to Bacteroidetes ratio, though this finding has not been universal across all studies (Ley et al., 2005, 2006). More consistent observations include reduced microbial diversity, decreased abundance of beneficial species such as Akkermansia muciniphila and Faecalibacterium prausnitzii, and increased abundance of bacteria associated with endotoxin production and metabolic dysfunction.
High-fat diet consumption rapidly alters gut microbiota composition, with changes detectable within 24 hours of dietary shift in animal models. These diet-induced changes include expansion of Firmicutes phyla, particularly Clostridiales and Erysipelotrichaceae families, and reduction of beneficial Bacteroidetes. Functionally, the obese-associated microbiota demonstrates enhanced capacity for energy harvest from diet (Turnbaugh et al., 2006), increased production of pro-inflammatory metabolites, and reduced production of beneficial metabolites like butyrate.
These microbial changes have direct consequences for gut-brain signaling. Studies have demonstrated that manipulating feeding patterns in high-fat diet fed rats can improve microbiota composition dynamics, reduce inflammation, and enhance gut-brain signaling. Specifically, manipulating meal timing and frequency, independent of total caloric intake, can partially restore beneficial microbiota, reduce intestinal permeability (Lam et al., 2012), decrease circulating inflammatory markers, and improve vagal afferent pathway structure and function. This suggests that both the composition of diet and the temporal pattern of feeding influence the microbiota-gut-brain axis.
The vagal afferent pathway is particularly susceptible to obesity-associated disruption. High-fat diet feeding leads to inflammatory cytokine production in nodose ganglia, structural alterations in vagal nerve fibers, and reduced vagal signaling efficacy (De Lartigue et al., 2011). These changes impair the transmission of satiety signals from the gut to the brain, contributing to the overconsumption of food despite adequate energy stores. Importantly, these vagal alterations are at least partially reversible through microbiota-targeted interventions, suggesting that vagal dysfunction in obesity represents a modifiable target rather than an irreversible consequence of obesity.
While obesity has received the most attention, alterations in the gut microbiota-brain axis contribute to other feeding and metabolic disorders. In type 2 diabetes, reduced microbial diversity and decreased abundance of butyrate-producing bacteria are consistently observed (Jiao et al., 2018). These changes are associated with impaired GLP-1 secretion, contributing to inadequate insulin release and reduced satiety signaling. Interventions that increase beneficial bacteria and butyrate production improve glucose control and can normalize eating patterns in diabetic individuals.
Non-alcoholic fatty liver disease (NAFLD), closely linked with obesity and insulin resistance, is also associated with specific microbiota signatures. Patients with NAFLD often show increased intestinal permeability, elevated circulating LPS levels, and altered bile acid metabolism due to dysbiosis. These changes can influence feeding behavior through effects on metabolic signaling and inflammatory pathways.
Stress-induced eating and emotional feeding behaviors, common in anxiety and depression, may also involve the microbiota-gut-brain axis. The gut microbiota influences the hypothalamic-pituitary-adrenal (HPA) axis, which regulates stress responses, and produces or modulates neurotransmitters including serotonin, gamma-aminobutyric acid (GABA), and dopamine (Bravo et al., 2011). Dysbiosis can heighten stress reactivity and promote emotional eating through these mechanisms. Emerging evidence suggests that certain probiotics, termed “psychobiotics,” can reduce anxiety and stress-related eating behaviors, though mechanisms require further elucidation.
Nutritional Interventions: From Bench to Bedside
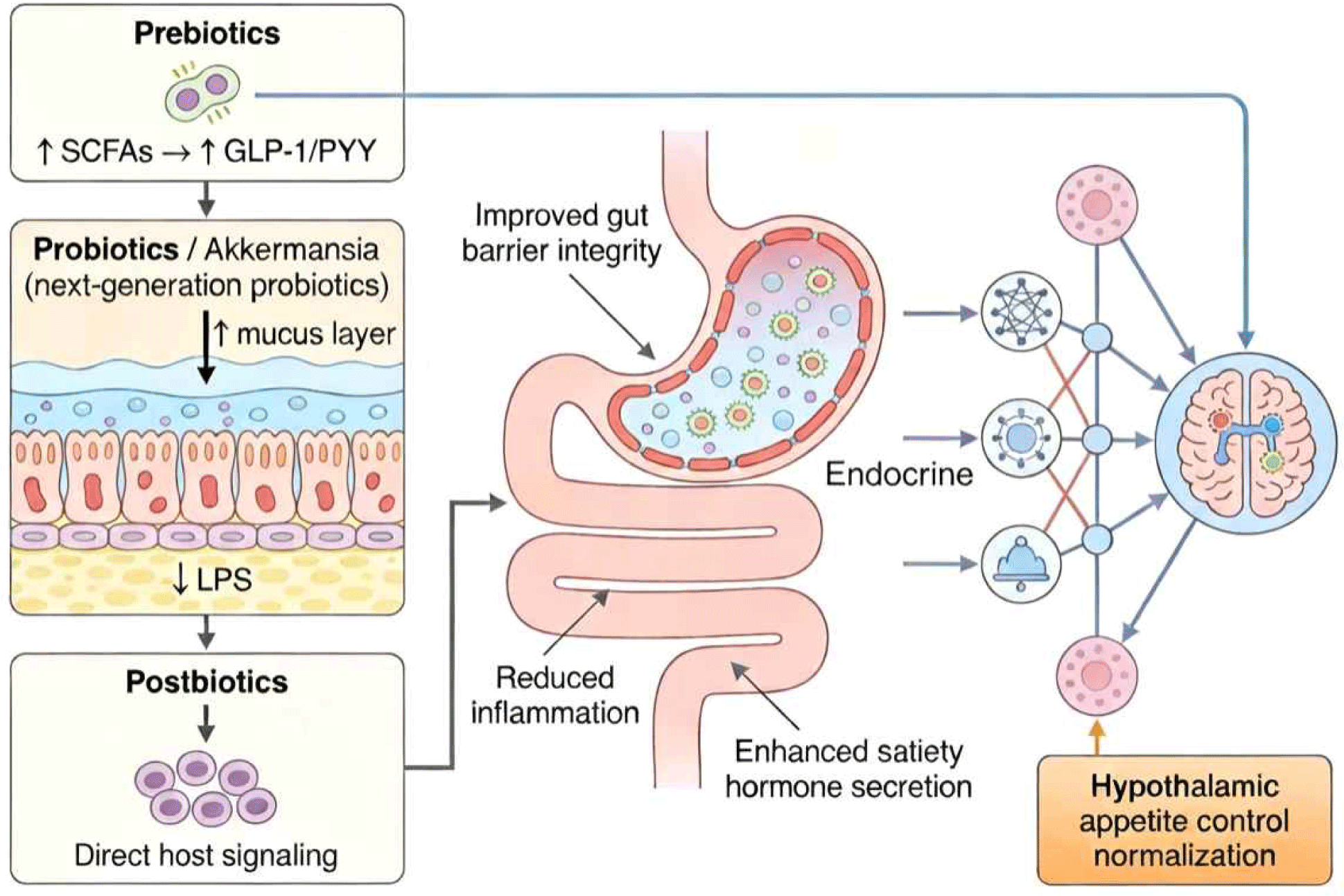
Targeting the gut microbiota through dietary interventions represents a promising, safe, and cost-effective strategy for modulating feeding behavior and improving metabolic health. Accumulating evidence from preclinical and clinical studies supports the efficacy of three major categories of interventions—prebiotics, probiotics, and postbiotics. Table 2 provides a structured summary of key microbiota-targeted nutritional interventions and their underlying mechanisms linking gut microbial modulation to the regulation of feeding behavior. In addition, Fig. 2 offers a schematic overview of how these interventions converge on gut–brain signaling pathways to influence appetite control and feeding behavior.
| Intervention category | Example | Model system | Key findings | Primary mechanisms | References |
|---|---|---|---|---|---|
| Prebiotics (inulin) | Inulin-type fructans | Overweight/obese humans (meta-analysis) |
↓Body weight (1-2 kg) ↓Fat mass ↑Satiety |
↑Bifidobacterium ↑SCFA production ↑GLP-1, PYY secretion |
Beserra et al., 2015 |
| Prebiotics (propionate ester) | Inulin-propionate ester | Overweight adults (RCT) |
↓Energy intake Prevention of weight gain ↓Reward brain activation |
↑Colonic propionate ↑PYY secretion Altered brain processing |
Chambers et al., 2015 |
| Prebiotic (GOS) | Galacto-oligosaccharides | Human adults (RCT) |
↓Cortisol response ↓Anxiety in IBS patients ↑Bifidobacterium |
↑SCFA production HPA axis modulation Microbiota shifts |
Schmidt et al., 2015 |
| Resistant starch | RS2/RS3 | Human adults (systematic review) |
↓Postprandial insulin ↑Insulin sensitivity ↑Satiety |
↑Butyrate production ↑GLP-1 secretion Improved glucose metabolism |
Bodinham et al., 2010 |
| Postbiotics | Butyrate | Low-fat & high-fat diet mice |
Cohort-dependent metabolic effects Variable glucose tolerance Microbiota-associated responsiveness |
Context-dependent SCFA signaling Baseline microbiota determines efficacy |
Lee et al., 2018 |
| Probiotics | Encapsulated probiotics | High-fat diet rats |
↑Barrier function ↓Inflammation Improved glucose metabolism Enhanced vs. non-encapsulated |
↑Intestinal delivery ↑Colonization ↓Metabolic endotoxemia |
Heo et al., 2019;
Lee et al., 2020 |
| Next-generation probiotics |
Pasteurized A. muciniphila |
Overweight/obese humans (RCT) |
↓Insulin resistance ↓Total cholesterol ↓Liver dysfunction markers Improved barrier function |
↑Mucus layer ↓Metabolic endotoxemia Immune modulation |
Depommier et al., 2019 |
| Functional foods | Goji berry | High-fat diet mice |
↑Intestinal integrity ↓Inflammatory profiles Improved gut microbiota ↓Body weight gain |
Prebiotic polysaccharides Anti-inflammatory polyphenols Microbiota modulation |
Jeong et al., 2024 |
[Note] The evidence summarized in this table spans preclinical studies, randomized controlled trials (RCTs), and meta-analyses, with greater interpretative weight given to human studies for clinical relevance. Preclinical findings were used primarily to support mechanistic understanding. Feeding behavior–related outcomes were interpreted based on their directness, with energy intake and satiety measures considered primary endpoints, followed by body weight/composition and metabolic markers as downstream outcomes. Stronger evidence for feeding behavior modulation was inferred when consistent effects were observed across multiple endpoint categories.
Prebiotics are defined as substrates that are selectively utilized by host microorganisms conferring a health benefit (Gibson et al., 2017). Unlike probiotics, which introduce live bacteria, prebiotics nourish existing beneficial microbiota, promoting their growth and metabolic activity. Several prebiotic compounds have shown particular promise for modulating the gut-brain axis and feeding behavior.
Inulin and fructo-oligosaccharides (FOS), derived from plants such as chicory root, Jerusalem artichoke, onions, and garlic, are among the most extensively studied and widely used prebiotics. These fructans consist of linear chains of fructose units and are classified based on chain length: FOS typically contains 2-10 fructose units, while inulin contains 10-60 units. Both resist digestion in the upper gastrointestinal tract and reach the colon intact, where they serve as selective substrates for beneficial bacteria.
Inulin and FOS selectively stimulate the growth of Bifidobacterium and Lactobacillus species, leading to increased production of short-chain fatty acids, particularly acetate, propionate, and butyrate (Cani et al., 2009). This increased SCFA production has multiple beneficial effects on feeding behavior and metabolism. Studies have demonstrated that inulin supplementation increases satiety hormone secretion, particularly GLP-1 and PYY, through SCFA-mediated activation of L-cells (Cani et al., 2009). In preclinical models, dietary supplementation with inulin reduces body weight gain, decreases fat mass accumulation, improves glucose tolerance, and reduces food intake.
While preclinical data are robust, human trials yield mixed results; Beserra et al. (2015) reported a weight loss of approximately 1–2 kg over 12 weeks with inulin supplementation, suggesting that efficacy may be limited without concurrent caloric restriction. A meta-analysis of randomized controlled trials demonstrated that inulin-type fructans significantly reduce body weight and fat mass in overweight and obese adults. The weight loss effects, while modest (typically 1-2 kg over 12 weeks), are achieved without caloric restriction, suggesting that appetite suppression through enhanced satiety signaling is a primary mechanism (Beserra et al., 2015).
The satiety-enhancing effects of inulin appear to involve multiple mechanisms beyond SCFA production. Inulin increases the viscosity of gastric contents, slowing gastric emptying and prolonging the sensation of fullness. Additionally, fermentation of inulin produces gases that may stimulate mechanoreceptors in the intestinal wall, contributing to satiety signaling. Recent studies have also demonstrated that inulin can modulate brain reward circuits, with one study showing that inulin-propionate ester supplementation reduced brain activation in reward-related regions during high-energy food cue exposure (Chambers et al., 2015).
Galacto-oligosaccharides are prebiotic fibers consisting of galactose units with a terminal glucose, produced commercially through enzymatic synthesis from lactose. GOS selectively stimulate the growth of Bifidobacterium and Lactobacillus species, leading to increased SCFA production, particularly acetate and butyrate. In preclinical models, GOS supplementation reduces body weight gain, improves glucose tolerance, and enhances satiety signaling (Cani et al., 2009).
Human studies have demonstrated that GOS supplementation can reduce the cortisol awakening response, suggesting stress-reducing effects that could influence stress-related eating behaviors (Schmidt et al., 2015). Additionally, GOS has been shown to reduce anxiety scores in patients with irritable bowel syndrome, a condition often associated with altered eating patterns. GOS-containing prebiotic mixtures have demonstrated beneficial effects on gut microbiota composition and metabolic parameters in multiple clinical trials.
The mechanisms by which GOS influences feeding behavior involve stimulation of SCFA production and subsequent activation of satiety pathways. GOS fermentation produces high levels of acetate and lactate, which can be further metabolized by cross-feeding bacteria to produce butyrate and propionate. This complex metabolic network contributes to the overall beneficial effects of GOS on gut-brain signaling and appetite regulation.
Resistant starch represents another important class of prebiotics that escape digestion in the small intestine and undergo bacterial fermentation in the colon. There are several types of resistant starch (RS1-RS5), categorized based on their structure and source. RS2 (found in raw potato and green banana) and RS3 (formed when starchy foods are cooked and cooled, such as in cooked and cooled rice or pasta) are particularly well-studied.
Resistant starch is a potent stimulator of butyrate production, often producing higher butyrate levels compared to inulin or FOS. This increased butyrate production has important implications for gut barrier function, as butyrate serves as the primary energy source for colonocytes and enhances tight junction protein expression (Hamer et al., 2008). Several studies have demonstrated that resistant starch supplementation improves insulin sensitivity, reduces postprandial glucose excursions, and increases satiety.
In human feeding studies, resistant starch supplementation has been shown to reduce subsequent meal energy intake and increase subjective feelings of fullness (Bodinham et al., 2010). A systematic review of controlled trials found that resistant starch consumption significantly reduces postprandial insulin responses and improves insulin sensitivity, effects that may contribute to improved appetite regulation and reduced risk of metabolic disease. The satiety-enhancing effects appear to be mediated primarily through SCFA production and subsequent GLP-1 secretion, though resistant starch may also have direct effects on gastric emptying and intestinal transit time.
Dietary polyphenols from fruits, vegetables, tea, coffee, and cocoa also function as prebiotics through their effects on gut microbiota composition and metabolic activity. While traditionally recognized for their antioxidant properties, polyphenols are now understood to exert significant effects through microbiota modulation. Most dietary polyphenols are poorly absorbed in the small intestine and reach the colon where they undergo microbial biotransformation into bioactive metabolites.
Polyphenols selectively promote the growth of beneficial bacteria such as Bifidobacterium and Lactobacillus while inhibiting pathogenic species (Molinari et al., 2022). The microbial metabolites produced from polyphenol degradation, including phenolic acids, can enhance gut barrier function, reduce inflammation, and improve metabolic parameters. Epidemiological studies have consistently associated high polyphenol intake with reduced obesity risk and improved metabolic health, though the relative contributions of direct polyphenol effects versus microbiota-mediated mechanisms remain under investigation.
The efficacy of prebiotics depends on several factors including dose, duration of intervention, baseline microbiota composition, and dietary context. Individual variability in response to prebiotics is substantial (Johnson et al., 2019), likely reflecting differences in microbiota composition and metabolic capacity. Individuals with lower baseline diversity or reduced abundance of prebiotic-fermenting bacteria may show enhanced responses compared to those with already-healthy microbiota profiles.
Dose-response relationships for prebiotics are complex, with higher doses not always producing superior outcomes. Very high doses of fermentable fibers can cause gastrointestinal discomfort including bloating, flatulence, and diarrhea, potentially reducing adherence. Most studies have used doses ranging from 5-20 g/day for inulin and FOS, with 10-15 g/day appearing to provide an optimal balance between efficacy and tolerability for most individuals.
Future research should focus on identifying biomarkers that predict prebiotic responsiveness to enable personalized interventions. Baseline microbiota profiling, combined with host genetic and metabolic phenotyping, may allow prediction of which individuals will benefit most from specific prebiotic interventions, moving toward precision nutrition approaches.
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit to the host. While traditional probiotic strains from Lactobacillus and Bifidobacterium genera have dominated the market, next-generation probiotics including Akkermansia muciniphila, Faecalibacterium prausnitzii, and specific Clostridium clusters are emerging as promising candidates for metabolic health applications.
Akkermansia muciniphila, a mucin-degrading bacterium residing in the intestinal mucus layer, has garnered significant attention due to its consistent inverse correlation with obesity, diabetes, and metabolic inflammation (Everard et al., 2013). Both live and pasteurized A. muciniphila supplementation improve metabolic parameters in preclinical models and humans. In a double-blind, placebo-controlled pilot study, daily supplementation with pasteurized A. muciniphila for three months reduced insulin resistance, decreased total cholesterol levels, and lowered markers of liver dysfunction and inflammation in overweight and obese participants (Depommier et al., 2019). These effects were associated with improved gut barrier function and reduced metabolic endotoxemia.
The mechanisms by which A. muciniphila exerts beneficial effects include strengthening the mucus layer, improving intestinal barrier integrity, modulating immune responses, and producing specific bioactive proteins. One such protein, Amuc_1100, an outer membrane protein, has been shown to recapitulate many of the beneficial effects of the whole bacterium (Plovier et al., 2017), suggesting potential for development as a postbiotic.
Lactobacillus and Bifidobacterium strains remain the most widely used probiotics and have demonstrated various metabolic benefits including improved glucose control, reduced inflammation, and modest weight loss effects. However, a significant limitation of oral probiotic administration is the harsh gastrointestinal environment, particularly the low pH of the stomach and bile salts in the small intestine, which can reduce bacterial viability and limit colonization of target intestinal regions.
Encapsulation strategies have been developed to protect probiotics from degradation and enhance their delivery to the colon. Studies have shown that beneficial effects of non-encapsulated or encapsulated probiotic supplementation on microbiota composition, intestinal barrier functions, inflammatory profiles, and glucose metabolism can differ (Lee et al., 2019), with encapsulation sometimes enhancing efficacy. Various encapsulation materials including alginate, chitosan, and resistant starch provide protection in the upper gastrointestinal tract and targeted release in the colon, potentially improving therapeutic outcomes.
A subset of probiotics termed “psychobiotics” has been defined as live organisms that, when ingested in adequate amounts, produce mental health benefits in patients with psychiatric illness. These strains, which include specific Lactobacillus and Bifidobacterium species, modulate the gut-brain axis through production of neurotransmitters (GABA, serotonin), regulation of the HPA axis, and reduction of inflammation (Bravo et al., 2011). Given the strong link between mood disorders and eating behaviors, psychobiotics may offer dual benefits for emotional regulation and appetite control. Clinical trials have reported that certain psychobiotic strains reduce perceived stress and anxiety (Messaoudi et al., 2011), which could secondarily improve stress-related eating patterns, though more research specifically examining feeding outcomes is needed.
Postbiotics, defined as preparations of inanimate microorganisms and/or their components that confer a health benefit on the host, represent an emerging category of microbiome-based therapeutics with several advantages over traditional probiotics. These advantages include improved safety profile (no risk of infection or antibiotic resistance gene transfer), enhanced stability during storage and transit through the gastrointestinal tract, easier standardization and quality control, and potentially reduced regulatory hurdles for clinical approval.
Direct supplementation with SCFAs, particularly butyrate, has been investigated as a postbiotic strategy for metabolic health. However, the relationship between SCFA supplementation and metabolic outcomes is complex and context-dependent. Studies examining metabolic responses to butyrate supplementation have revealed significant heterogeneity in outcomes that are associated with baseline gut microbiota composition and function. In one study, metabolic responses to butyrate supplementation in low-fat and high-fat fed mice were cohort-dependent and associated with changes in composition and function of the gut microbiota (Lee et al., 2020). Mice that responded positively to butyrate exhibited distinct microbiota signatures at baseline compared to non-responders, suggesting that microbiota composition determines the efficacy of SCFA supplementation (Sanna et al., 2019).
This finding has important implications for clinical translation. Rather than adopting a one-size-fits-all approach, SCFA-based interventions may need to be personalized based on individual microbiota profiles. Additionally, dietary delivery of SCFAs (e.g., inulin-propionate ester) may be more effective than direct supplementation (Chambers et al., 2015), as it delivers SCFAs specifically to the colon where they exert their primary effects and avoids rapid absorption and metabolism that occurs with oral SCFA administration.
Inactivated bacteria, produced through heat treatment, UV irradiation, or other methods, retain many of the beneficial immunomodulatory and metabolic effects of live bacteria while offering improved safety and stability. Studies in both animal models and humans have demonstrated that pasteurized probiotics can be as effective as live bacteria for improving metabolic parameters (Depommier et al., 2019).
Specific bacterial components including outer membrane proteins, exopolysaccharides, and cell wall fragments also function as postbiotics. The aforementioned Amuc_1100 protein from A. muciniphila improves metabolism in obese and diabetic mice through mechanisms involving improved gut barrier function and reduced inflammation (Plovier et al., 2017). Similarly, bacterial cell wall components and metabolites from various Lactobacillus species have been shown to prevent obesity and improve glucose metabolism in high-fat diet-fed mice.
A systematic review and meta-analysis of randomized controlled trials examining postbiotic supplementation effects on obesity and metabolic health demonstrated significant beneficial effects on metabolic parameters (Vallianou et al., 2020). Animal studies have shown even more pronounced effects, with postbiotic supplementation reducing body weight, body fat, and improving lipid and glycemic profiles. The anti-obesity effects of postbiotics appear to involve multiple mechanisms including increased energy expenditure, reduced adipogenesis, suppression of food intake through enhanced satiety hormone secretion, inhibition of dietary lipid absorption, and favorable modulation of gut microbiota composition.
While preclinical evidence for postbiotics is robust, clinical data remain limited but growing. Recent trials have demonstrated that postbiotic supplementation can improve metabolic parameters in individuals with obesity and metabolic syndrome, with safety profiles superior to probiotics. However, standardization of postbiotic preparations, optimal dosing strategies, and identification of responsive populations remain challenges requiring further investigation.
While isolated prebiotics, probiotics, and postbiotics offer targeted interventions, whole food approaches that naturally modulate the gut microbiota may provide additional benefits through synergistic effects of multiple bioactive compounds.
Fermented foods have been consumed for millennia and are experiencing renewed interest for their potential to improve gut health and metabolic parameters. These foods deliver live bacteria along with bioactive metabolites produced during fermentation, functioning as natural synbiotics (Han et al., 2015).
Goji berry (Lycium barbarum), a traditional medicinal plant rich in polysaccharides, polyphenols, and carotenoids, has demonstrated prebiotic potential in recent studies. Supplementation with goji berry in high-fat diet-fed mice improved intestinal integrity and inflammatory profiles via modification of the gut microbiota (Jeong et al., 2024). Specifically, goji berry increased the abundance of beneficial bacteria, reduced markers of intestinal inflammation, and improved tight junction protein expression. These effects were associated with improved metabolic parameters including reduced body weight gain and improved glucose tolerance. Both non-fermented and fermented goji berry preparations showed benefits (Lee et al., 2021), with fermentation potentially enhancing bioavailability of certain bioactive compounds. These findings suggest that goji berry and similar functional foods could serve as accessible dietary interventions for obesity prevention and metabolic health promotion.
Red ginseng, another traditional Asian medicine with immunomodulatory and anti-fatigue properties, has also been investigated for metabolic effects. Supplementation with non-fermented and fermented red ginseng improved obese phenotypes, lipid and inflammatory profiles, and antioxidant defense systems in high-fat fed rats, with effects mediated partially through gut microbiota modulation (Eun et al., 2023).
Kimchi, a traditional Korean fermented vegetable dish, has been extensively studied for its probiotic and prebiotic properties. Daily consumption of kimchi has been shown to alter gut microbiota composition and improve metabolic syndrome markers in obese women. The effects of kimchi involve both the live lactic acid bacteria present in the fermented product and the fiber and bioactive compounds from the vegetables.
While this review focuses on microbiota-mediated mechanisms, it is important to acknowledge that many functional foods contain bioactive compounds that exert direct metabolic effects independent of microbiota modulation. For example, anthocyanins and other polyphenols from various plant sources have been shown to possess potent antioxidant and anti-inflammatory properties. These compounds may work synergistically with microbiota-mediated effects to provide comprehensive metabolic benefits. Understanding the relative contributions of microbiota-dependent and -independent mechanisms is an important area for future research.
Feeding Pattern Manipulation: An Overlooked Strategy
While most research on the gut-brain axis and feeding behavior has focused on the composition of diet and microbiota-targeted supplements, the temporal pattern of food intake represents an underexplored but potentially powerful intervention strategy. The timing, frequency, and regularity of meals influence gut microbiota composition and activity, which in turn affect metabolic health and feeding regulation through gut-brain signaling pathways (Chevalier et al., 2015).
Circadian rhythms govern numerous physiological processes including metabolism, immune function, and feeding behavior. The gut microbiota itself exhibits diurnal oscillations in composition and metabolic activity that are entrained by feeding patterns (Chevalier et al., 2015). Disruption of these rhythms through irregular eating schedules, shift work, or constant food availability contributes to metabolic dysfunction and obesity.
Time-restricted feeding (TRF), in which food intake is confined to a consistent window of 8-12 hours per day without necessarily reducing total caloric intake, has emerged as an effective intervention for obesity and metabolic disease. While the metabolic benefits of TRF were initially attributed primarily to effects on circadian clock genes and metabolic signaling, recent evidence indicates that gut microbiota plays a crucial role in mediating these effects (Zarrinpar et al., 2014).
Studies have demonstrated that manipulation of feeding patterns in high-fat diet fed rats improves microbiota composition dynamics, inflammation, and gut-brain signaling. Specifically, implementing a consistent feeding schedule, even without caloric restriction, led to several beneficial outcomes: increased abundance of beneficial bacteria including Akkermansia and butyrate-producing species, reduced intestinal permeability as evidenced by decreased circulating LPS levels (Lam et al., 2012), decreased pro-inflammatory cytokine production (TNF-α, IL-1β, IL-6) in both peripheral tissues and central nervous system, improved structure and function of the vagal afferent pathway with reduced inflammatory infiltration in nodose ganglia, and enhanced hypothalamic leptin sensitivity. These effects collectively contributed to improved appetite regulation and reduced hyperphagia despite continued high-fat diet consumption.
The mechanisms underlying these benefits involve restoration of circadian alignment in both host and microbiota. Regular feeding times entrain microbial metabolic activity, leading to rhythmic production of SCFAs and other metabolites that synchronize host circadian clocks. This alignment optimizes metabolic efficiency, enhances gut barrier function during feeding periods, and improves the fidelity of gut-brain signaling (Thaiss et al., 2014).
The interaction between feeding patterns and microbiota-targeted interventions represents an exciting area for future research. Emerging evidence suggests that the efficacy of prebiotics, probiotics, and dietary interventions may depend on when they are consumed relative to feeding windows and circadian phase. For example, consuming prebiotics during the active feeding period when microbial fermentation is most robust may enhance SCFA production and subsequent metabolic benefits.
Combining feeding pattern manipulation with microbiota-targeted supplements could produce synergistic effects. The structured feeding approach establishes favorable conditions for beneficial microbiota, while prebiotic or probiotic supplementation actively cultivates these populations. This multimodal approach may be particularly effective for individuals with severe dysbiosis or metabolic dysfunction.
Importantly, feeding pattern manipulation represents a cost-free intervention that can be implemented immediately without requiring specialized foods or supplements. For populations with limited access to healthcare or inability to afford functional foods, educating individuals about the importance of regular meal timing could provide significant metabolic benefits. However, adherence to structured eating schedules can be challenging in modern society with variable work schedules, social obligations, and food availability. Developing practical strategies to implement and maintain consistent feeding patterns in real-world settings is an important direction for translational research.
Emerging Technologies and Future Directions
The field of microbiota-gut-brain axis research is rapidly evolving, driven by technological innovations that enable more sophisticated mechanistic investigations and accelerated translation to clinical applications.
Traditional approaches to studying the gut-brain axis have relied heavily on animal models, which, while informative, have limitations in translating findings to humans due to species differences in microbiota composition, physiology, and feeding behavior. Emerging technologies are addressing these limitations.
Organ-on-a-chip devices represent a paradigm shift in modeling complex physiological systems. These microfluidic platforms recreate the mechanical, chemical, and cellular microenvironment of tissues, enabling investigation of human-relevant biological processes in vitro. Gut-on-a-chip models have been developed that incorporate intestinal epithelial cells, immune cells, and gut microbiota in a dynamic system that mimics peristaltic movements and fluid flow.
Recent advances have enabled integration of neural components into gut-on-a-chip systems, creating platforms for studying gut-brain axis interactions. These models can incorporate enteric neurons, enteroendocrine cells, and even sensory neurons to investigate how microbial metabolites influence neural signaling. Such systems offer several advantages: use of human cells enabling species-specific responses, ability to isolate and manipulate specific variables (e.g., individual bacterial species or metabolites), real-time monitoring of barrier integrity, hormone secretion, and neural activity, high-throughput screening capability for identifying beneficial bacteria or metabolites, and reduced reliance on animal experimentation.
Applications of gut-on-a-chip models for studying gut-brain axis and appetite regulation are expanding rapidly. These platforms are being used to identify bacterial strains that enhance GLP-1 secretion, investigate mechanisms of SCFA-induced satiety signaling, study how inflammatory mediators affect enteric neuron function, and screen potential prebiotic and postbiotic compounds for efficacy. As these technologies mature, they promise to accelerate discovery of novel microbiota-targeted interventions for feeding behavior disorders.
Intestinal organoids—three-dimensional cultures of intestinal stem cells that self-organize into structures resembling native intestinal epithelium—complement organ-on-a-chip approaches. Organoids can be derived from patient biopsies, enabling personalized modeling of gut-microbiota-host interactions. Combining organoids with co-culture of specific bacterial strains allows investigation of how individual microbiota components influence epithelial barrier function, hormone secretion, and immune signaling. Integration of organoid and organ-on-a-chip technologies may ultimately enable creation of multi-organ systems linking gut, liver, and brain tissue to model systemic effects of gut-derived signals on metabolism and feeding behavior.
Individual variability in response to dietary interventions is substantial and represents a major challenge for developing effective nutrition-based therapies. Precision nutrition—the tailoring of dietary recommendations to individual characteristics including genetics, microbiome composition, metabolic phenotype, and lifestyle factors—offers a path forward (Johnson et al., 2019).
Microbiome-based precision nutrition relies on characterizing an individual’s gut microbiota composition and functional capacity to predict responses to specific foods or supplements. Several companies and research groups have developed algorithms that analyze microbiome sequencing data along with other biomarkers to generate personalized dietary recommendations. Proof-of-concept studies have demonstrated that such approaches can improve glycemic control more effectively than standard dietary advice (Zeevi et al., 2015).
For feeding behavior applications, precision nutrition could identify individuals most likely to benefit from specific prebiotics, probiotics, or feeding pattern interventions based on their baseline microbiota and metabolic profiles. For example, individuals with low abundance of butyrate-producing bacteria might be prioritized for interventions that increase these species, while those with compromised gut barrier function might benefit most from interventions targeting intestinal integrity.
Challenges for precision nutrition include the cost and complexity of multi-omics profiling, limited understanding of causal relationships between microbiota and metabolic outcomes, temporal variability in microbiota composition, and need for large, diverse datasets to train and validate prediction algorithms. Despite these challenges, the field is advancing rapidly, and precision nutrition approaches are likely to become increasingly accessible and effective in the coming years.
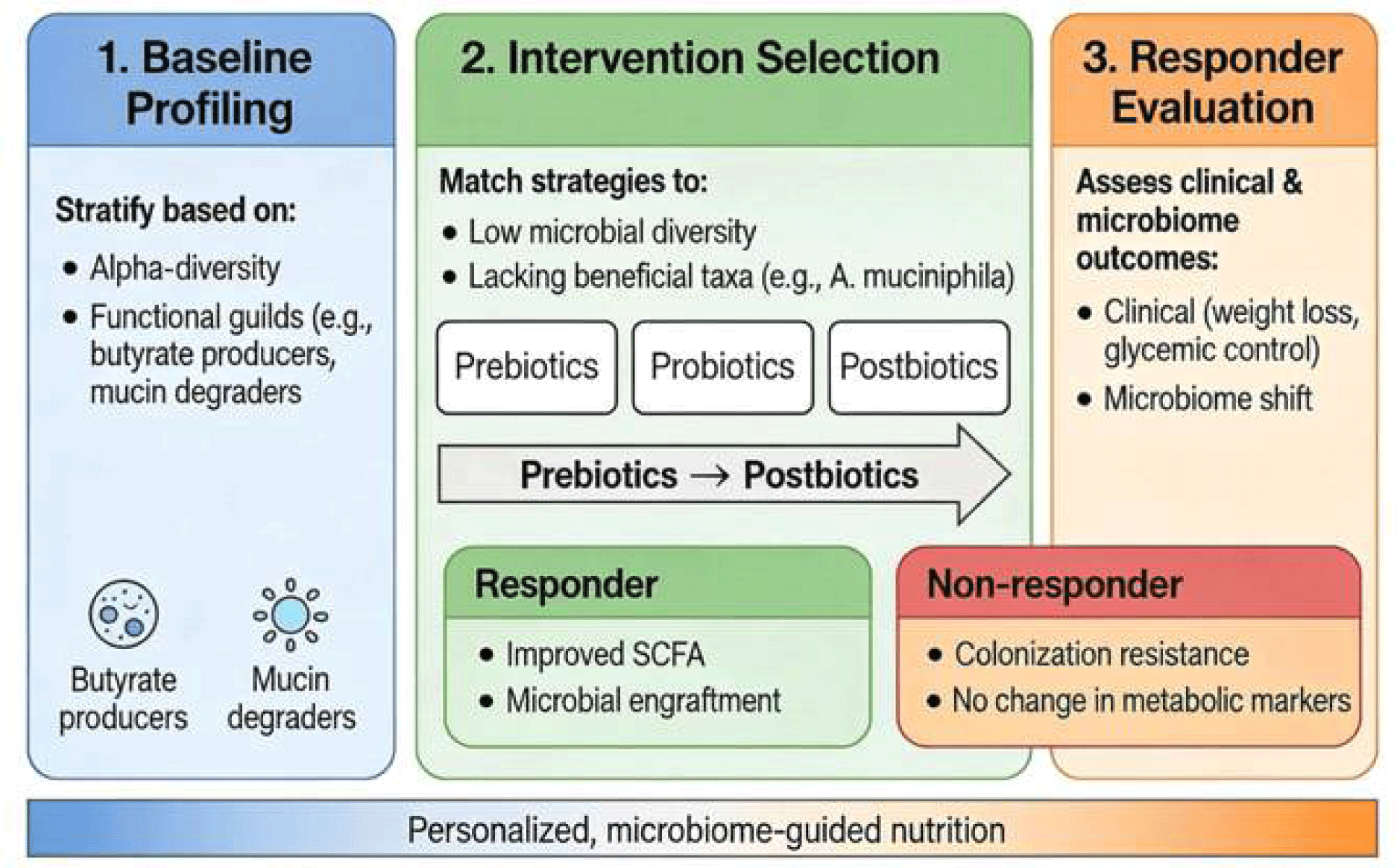
To operationalize this, we propose a conceptual framework for microbiota-guided interventions consisting of three phases (Fig. 3): 1. Baseline Profiling: Individuals are stratified based on key microbiome features, such as alpha-diversity and the presence of specific functional guilds (e.g., butyrate producers or mucin degraders). 2. Intervention Selection: Strategies are matched to these profiles; for instance, individuals with low microbial diversity may prioritize prebiotics or high-fiber diets to expand the metabolic niche, whereas those lacking specific beneficial taxa (e.g., Akkermansia muciniphila) would be candidates for targeted probiotics or postbiotics. 3. Responder Evaluation: Post-intervention assessment integrates clinical endpoints (e.g., weight loss, glycemic control) with microbiome shifts. True “responders” are defined by the convergence of clinical improvement and successful microbial engraftment or metabolic activation (e.g., increased SCFA production), enabling the differentiation of non-responders due to resistance to colonization versus lack of host physiological response.”
While preclinical evidence for microbiota-targeted interventions is compelling, several challenges must be addressed to achieve widespread clinical implementation.
Effects of probiotics are highly strain-specific, with different strains of the same species often producing distinct or even opposing effects. This specificity necessitates rigorous characterization of bacterial strains used in interventions and careful attention to quality control during manufacturing. Additionally, regulatory frameworks for probiotics vary widely across countries, and standards for demonstrating efficacy and safety are not always clearly defined. Development of consensus guidelines for probiotic research and commercialization would facilitate clinical translation.
For postbiotics, defining the active components and establishing dose-response relationships is essential. Unlike live bacteria, which can proliferate in the gut, postbiotics require delivery of sufficient quantities of active compounds to exert effects. Understanding the bioavailability and pharmacokinetics of different postbiotic preparations is an important area for future research.
Optimal doses and treatment durations for microbiota-targeted interventions are not well established. Most clinical trials have used empirically chosen doses based on preclinical studies or manufacturer recommendations, but systematic dose-finding studies are rare. Similarly, the required duration of treatment to achieve sustained benefits is unclear. Some studies suggest that microbiota changes induced by prebiotics or probiotics revert to baseline shortly after discontinuation, raising questions about the need for continuous versus intermittent dosing.
While generally regarded as safe, the long-term effects of chronic microbiota manipulation are not fully understood. Concerns include potential disruption of commensal microbiota balance, selection for antibiotic resistance, and unintended metabolic consequences. Long-term clinical trials with extended follow-up periods are needed to address these safety questions and determine whether metabolic benefits are sustained over years of treatment.
Most individuals with obesity or metabolic disorders receive multiple interventions including dietary counseling, pharmacological treatments (e.g., metformin, GLP-1 receptor agonists), and sometimes bariatric surgery. Understanding how microbiota-targeted interventions interact with these standard treatments is important for safe and effective implementation. Some evidence suggests that prebiotics and probiotics may enhance the efficacy of certain medications, while in other cases, interactions could reduce effectiveness. Systematic evaluation of combination therapies is needed.
Despite significant progress, important questions remain unanswered:
Causality versus Correlation: While numerous associations between microbiota composition and feeding behavior or metabolic phenotypes have been identified, establishing causality requires rigorous experimental approaches (Alcock et al., 2014). Mechanistic studies using gnotobiotic animals, fecal microbiota transplantation, and defined bacterial consortia are needed to definitively determine which bacteria and metabolites causally influence feeding behavior.
Critical Windows for Intervention: Early-life microbiota colonization influences long-term metabolic health, but the extent to which adult microbiota can be durably modified and whether critical periods exist for intervention remain unclear. Understanding developmental trajectories of the microbiota-gut-brain axis could identify optimal times for preventive interventions.
Sex Differences: Most microbiota research has been conducted in male rodents or mixed-sex human cohorts without stratified analysis. Emerging evidence suggests sex differences exist in microbiota composition, metabolic responses to diet, and gut-brain signaling. Incorporating sex as a biological variable in future studies is essential for understanding these differences and developing sex-specific interventions when appropriate.
Mechanisms of Individual Variability: Why do individuals respond differently to identical dietary interventions (Johnson et al., 2019)? Beyond microbiota composition, factors including host genetics, epigenetics, immune status, concurrent medications, stress levels, and physical activity all likely contribute. Integrative systems biology approaches that consider multiple levels of biological organization are needed to understand and ultimately predict individual responses.
Conclusions and Perspectives
The gut microbiota-brain axis has emerged as a critical regulator of feeding behavior, operating through multiple synergistic communication pathways that integrate peripheral metabolic signals with central neural circuits controlling appetite, satiety, and energy homeostasis. Neural signaling via the vagal afferent pathway provides rapid transmission of satiety signals from gut to brain (De Lartigue, 2016), with microbiota influencing both the structure and function of these neural connections. Endocrine signaling through gut hormones, particularly GLP-1 and PYY, mediates longer-lasting satiety effects (Martin et al., 2019), with microbial metabolites acting as potent stimulators of hormone secretion. Metabolic signaling via short-chain fatty acids produced from bacterial fermentation directly influences energy balance through effects on multiple tissues including intestinal epithelium, liver, adipose tissue, and brain (Canfora et al., 2015). Immune signaling links gut barrier integrity and metabolic inflammation with hypothalamic feeding circuits (Cani et al., 2007), with dysbiosis-induced endotoxemia contributing to leptin resistance and hyperphagia.
Dysbiosis of the gut microbiota disrupts these finely tuned regulatory systems, contributing to obesity and related feeding disorders (Ley et al., 2006; Turnbaugh et al., 2006). However, the modifiable nature of the gut microbiota presents therapeutic opportunities. Nutritional interventions targeting the microbiota—including prebiotics such as inulin, fructo-oligosaccharides, galacto-oligosaccharides, and resistant starch (Beserra et al., 2015; Cani et al., 2009; Chambers et al., 2015), probiotics both traditional and next-generation (Depommier et al., 2019), and emerging postbiotics including SCFAs and inactivated bacteria (Plovier et al., 2017)—have demonstrated efficacy in preclinical models and show promise in clinical trials. These interventions improve gut barrier integrity, enhance satiety hormone secretion, reduce inflammation, and optimize gut-brain signaling through the vagal pathway and other routes. Notably, whole food approaches using functional foods like goji berry (Lee et al., 2021) provide accessible, culturally acceptable vehicles for delivering multiple bioactive compounds simultaneously.
Feeding pattern manipulation represents an underexplored yet powerful complementary strategy that can optimize the gut microbiota-brain axis without requiring specialized supplements or foods (Chevalier et al., 2015; Zarrinpar et al., 2014). Implementing consistent meal timing enhances circadian alignment of microbial and host metabolism, improves gut barrier function, and strengthens gut-brain signaling pathways. The synergy between dietary composition, microbiota-targeted supplements, and feeding patterns offers opportunities for multimodal interventions with potentially superior efficacy compared to any single approach.
Emerging technologies including gut-on-a-chip models and intestinal organoids promise to accelerate mechanistic discovery and enable screening of novel interventions in human-relevant systems. These advanced platforms can reveal how specific bacterial strains and metabolites influence gut-brain signaling at cellular and molecular levels, facilitating identification of next-generation probiotics and postbiotics. Integration of multi-omics approaches—combining microbiome sequencing, metabolomics, transcriptomics, and other data types—with machine learning algorithms is paving the way toward precision nutrition (Johnson et al., 2019; Zeevi et al., 2015), in which dietary interventions are tailored to individual microbiota profiles and metabolic phenotypes.
Clinical translation of microbiota-based interventions faces challenges including establishing optimal doses and durations, accounting for individual variability in response, ensuring long-term safety, and integrating with existing therapies. However, the growing clinical evidence base, particularly from randomized controlled trials demonstrating metabolic benefits of prebiotics and next-generation probiotics (Beserra et al., 2015; Chambers et al., 2015; Depommier et al., 2019), supports the therapeutic potential of this approach. As standardization improves, costs decrease, and regulatory frameworks mature, microbiota-targeted interventions are likely to become important components of obesity prevention and treatment strategies.
Looking forward, several priorities emerge. First, mechanistic research must continue to elucidate the causal relationships between specific microbial species, metabolites, and feeding behavior outcomes. Second, large-scale, long-duration clinical trials are needed to establish the efficacy and safety of microbiota interventions across diverse populations. Third, development of biomarkers that predict individual responses to interventions will enable precision nutrition approaches. Fourth, investigation of critical windows for intervention, particularly during early development, may identify opportunities for prevention. Fifth, integration of microbiota-based strategies with behavioral and pharmacological approaches may produce synergistic benefits exceeding any single intervention.
The microbiota-gut-brain axis represents a paradigm shift in our understanding of feeding behavior regulation, revealing that the trillions of microorganisms inhabiting our gastrointestinal tract are active participants in the neural and metabolic circuits controlling when, what, and how much we eat (Fetissov, 2017; Torres-Fuentes et al., 2017). Harnessing this biology through targeted nutritional interventions offers a safe, accessible, and potentially transformative approach to addressing the obesity epidemic and improving metabolic health on a population scale.
