INTRODUCTION
Alzheimer’s disease (AD) is the most common form of dementia, which is closely connected with systemic inflammation including neuroinflammation and neurotransmitter imbalance (Cao et al., 2020; Tarawneh and Holtzman, 2012). In particular, neuronal inflammation is known to be deteriorated by the alteration of gut microbiota and overexpression of bacterial toxins such as lipopolysaccharide (LPS) (Busche and Hyman, 2020; Cryan et al., 2020; Jang et al., 2018; Reitz and Mayeux, 2014). Exposure to gut bacteria LPS causes neuroinflammation and gut inflammation in rodents, resulting in cognitive impairment (CI) through the suppression of BDNF expression (Jang et al., 2018). BDNF regulates synapse in the many brain regions including hippocampus for maintaining memory storages (Afonso et al., 2019; Lu et al., 2014).
Approximately 40% of patient with gut inflammation suffer from psychiatric diseases with gut dysbiosis (Almeida et al., 2020; Fousekis et al., 2021). The breaking of gut microbiota by antibiotics, pathogens, and stresses, causes the overgrowth of harmful bacteria such as Clostridioides difficile and toxic substances such as endotoxins (Dodiya et al., 2020; Konstantinidis et al., 2020). Chronic exposure to overproduced endotoxins occurs gut and systemic inflammation, resulting in psychiatric disorder (Jang et al., 2018; Marizzoni et al., 2020). Therefore, the alleviation of gut dysbiosis by probiotics lessens the endotoxin overexpression, leading to the amelioration of gut and systemic inflammation (Angelucci et al., 2019; Gomaa, 2020; Westfall et al., 2017). Gut bacteria lipopolysaccharide (LPS) production- inhibiting Lactobacillus plantarum NK151 alleviates CI with gut inflammation in mice (Lee et al., 2021b). Anti- inflammatory Bifidobacterium longum NK46 improves CI with colitis in rodents (Lee et al., 2019). IL-1β expression- inhibiting Lactobacillus gasseri NK109 also increases cognitive function in aged or Escherichia coli-treated mice (Yun et al., 2020; Yun et al., 2023). Anti- inflammatory Lactiplantibacillus plantarum C29 increases cognitive function in 5xFAD and aged mice by upregulating inflammation-mediated brain-derived neurotropic factor (BDNF) expression (Jeong et al., 2015; Lee et al., 2018). Based on these findings, up-regulating NF-κB activation- suppressed BDNF expression may be beneficial for the therapy of cognitive impairment. Furthermore, the cognitive impairment-ameliorating effects of probiotics are enhanced by the addition of natural products such as soybean (Glycine max), which also improves cognitive impairment (Hwang et al., 2019; Lee et al., 2021a; Lee et al., 2018). Nevertheless, the cognitive impairment- ameliorating action mechanism of NK109 with or without natural products remains elusive.
Therefore, to confirm whether the suppression of NF-κB signaling by probiotics could regulate CI, we examined the effects of Lactobacillus gasseri NK109 and its supplement combined with soybean embryo ethanol extract (SE) on LPS-induced CI in mice.
MATERIALS AND METHODS
LPS and MRS were purchased from Sigma (St Louis, MO) and BD (Franklin Lakes, NJ), respectively. A commercial soybean germ ethanol extract (SE, unprocessed, contained 0.2% soyasaponin Bb) was purchased from Mirae Biotech (Pocheon-shi, Korea).
NK109, which was isolated from human feces (female, 34 yrs, Korean) in the previous report (Yun et al., 2020), was cultured in MRS broth at 37°C for 24 h, centrifuged, and freeze-dried.
Macrophage cells (peritoneal macrophages and BV2 cells) were cultured, as previously reported (Jeong et al., 2016; Lee et al., 2017). These cells (1 × 106 cells/mL) were incubated with LPS (100 ng/mL) in the absence or presence of NK109 (N4, 1 × 104 CFUs/mL; N6, 1 × 106 CFUs/mL) for 20 h. In the supernatant, proinflammatory cytokine levels were assayed.
C57BL/6 mice (male, 6 weeks old, 18–20 g) were purchased from Koatech (Pyeongtaek-shi, Korea), maintained under the controlled condition, as previously reported. All experiments were approved by the Committee for the Care and Use of Laboratory Animals at Kyung Hee University (IACC, KHUASP(SE)-21306) and were ethically carried out according to the University Guideline.
Mice with CI were prepared by intraperitoneally injecting LPS (10 μg/kg/day), as previously reported (Hong et al., 2014; Lee et al., 2011). (1) Mice were divided into four groups (NC, LP, NL, and NH), intraperitoneally injected with LPS daily for 5 days. Test agents (LP, vehicle alone; NL, 5 × 108 CFU/mouse of NK109; NH, 1 × 109 CFU/mouse of NK109) were orally gavaged daily for 5 days next day after LPS treatment. Each group consisted of 7 mice. (2) Mice were divided into five groups (NC, LP, NL, SENL, and SENH), intraperitoneally injected with LPS daily for 5 days. Thereafter, test agents (LP, vehicle alone; SENL, the mixture of 100 mg/kg/day of SE and 0.5 × 109 CFU/mouse/day of NK109; and SENH, the mixture of 100 mg/kg/day of SE and 1 × 109 CFU/mouse/day of NK109) were orally gavaged daily for 5 days. NC was treated with saline instead of LPS and test agents. Each group consisted of 7 mice.
Cognitive behaviors were measured in the Y-maze task (YMT), novel object recognition test (NORT), and Barnes maze task (BMT) (Yun et al., 2021; Yun et al., 2023). Mice was killed by exposure to CO2 in a chamber. Bloods, brains, and colons were collected and stored at – 80°C. The biomarkers were assayed using enzyme-linked immunosorbent assay (ELISA). For the immunostaining, mice were perfused and brain and colon tissues were collected, post-fixed with paraformaldehyde, cytoprotected, freezed, immunostained, and observed using a confocal microscope.
RESUTLS
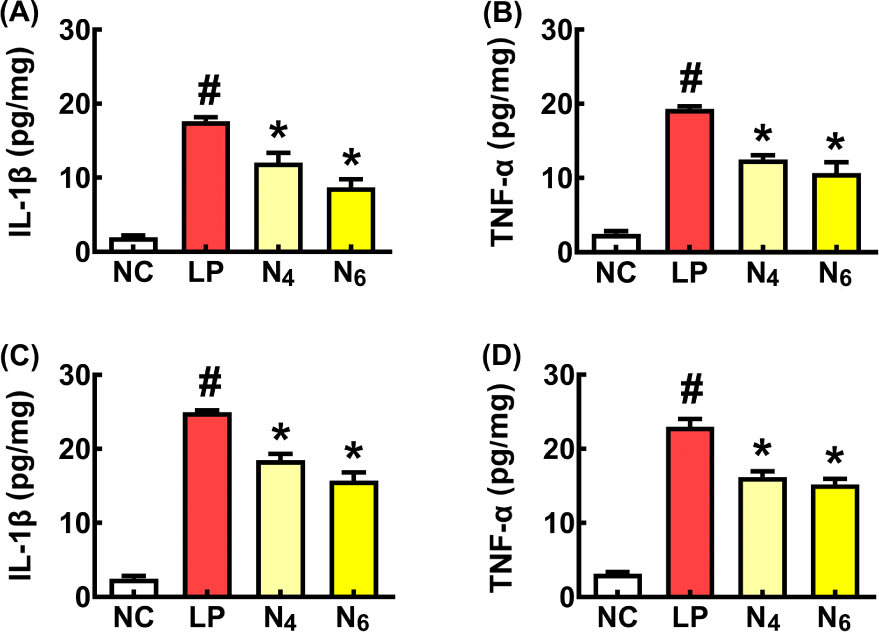
To understand whether NK109 could mitigate inflammation-involved cognitive decline, we first investigated the effect of NK109 on proinflammatory cytokine expression in LPS-stimulated macrophages (Fig. 1). LPS treatment significantly up-regulated TNF-α and IL-1β expression in peritoneal macrophages and BV2 cells. However, NK109 inhibited aLPS-induced TNF-α and IL-1β expression.
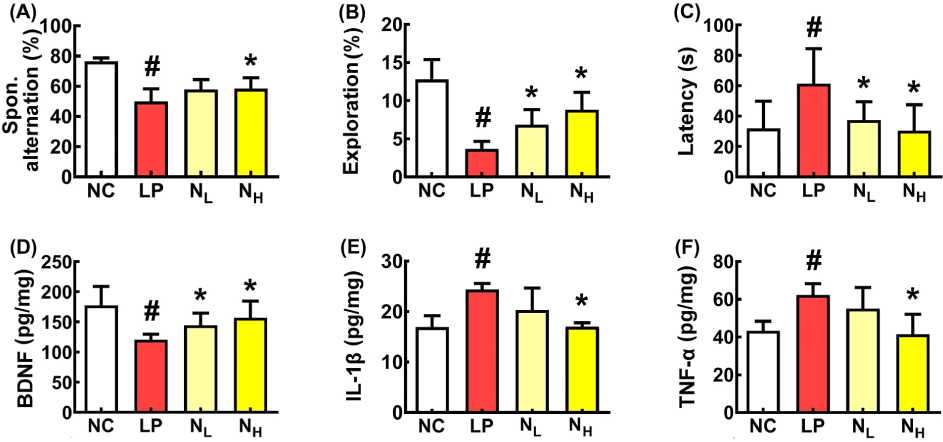
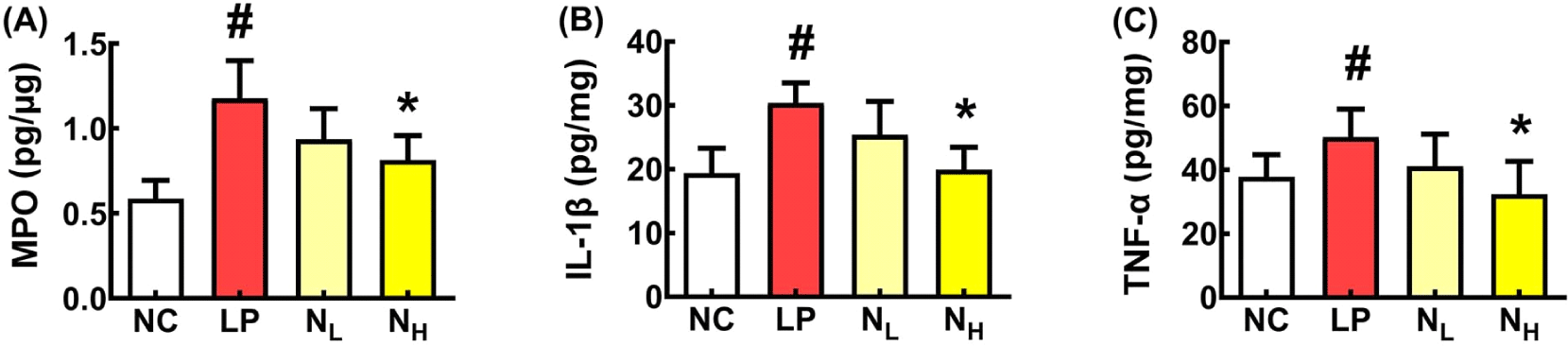
Next, we investigated the effect of NK109 on LPS-induced CI in mice (Fig. 2). Intraperitoneally injected LPS impaired cognitive behaviors in the YMT to 65.2% of normal control (NC) mice. However, oral administrations of NK109 improved LPS-impaired cognitive function. NK109 at a dose of 50 mg/kg (NL) restored LPS-impaired cognitive function to 89.8% of NC mice. However, LPS or NK109 did not significantly affect average values of the arm entry numbers in the YMT. LPS treatment also reduced exploration in the NORT to 28.9% of NC mice and increased the escape time (on the third day) in the BMT to 192.8% of NC mice. However, NK109 alleviated exploration and latency. NL treatment increased LPS-impaired exploration time to 58.9% of NC mice and decreased LPS-induced escape time to 115.1% of NC mice. The CI-ameliorating effect of NH was higher without significance than that of NL. Furthermore, NK109 suppressed IL-1β and TNF-α expression in the brain, whereas BDNF expression increased. NK109 also decreased LPS-induced myeloperoxidase, IL-1β, and TNF-α expression in the colon (Fig. 3).
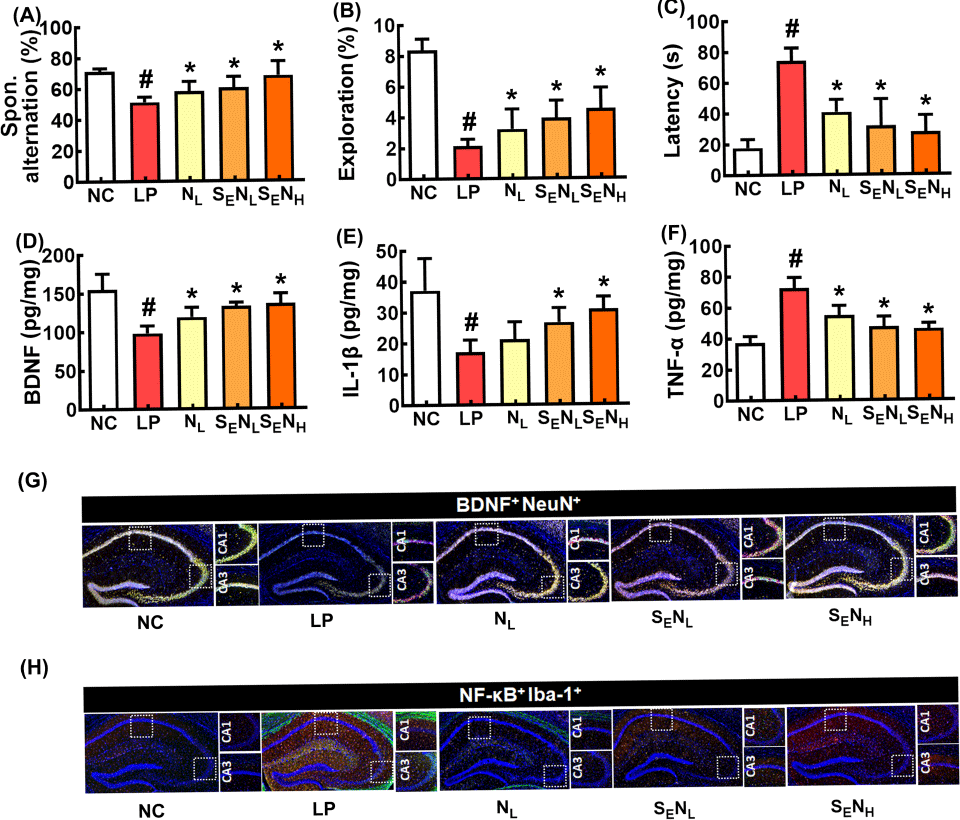
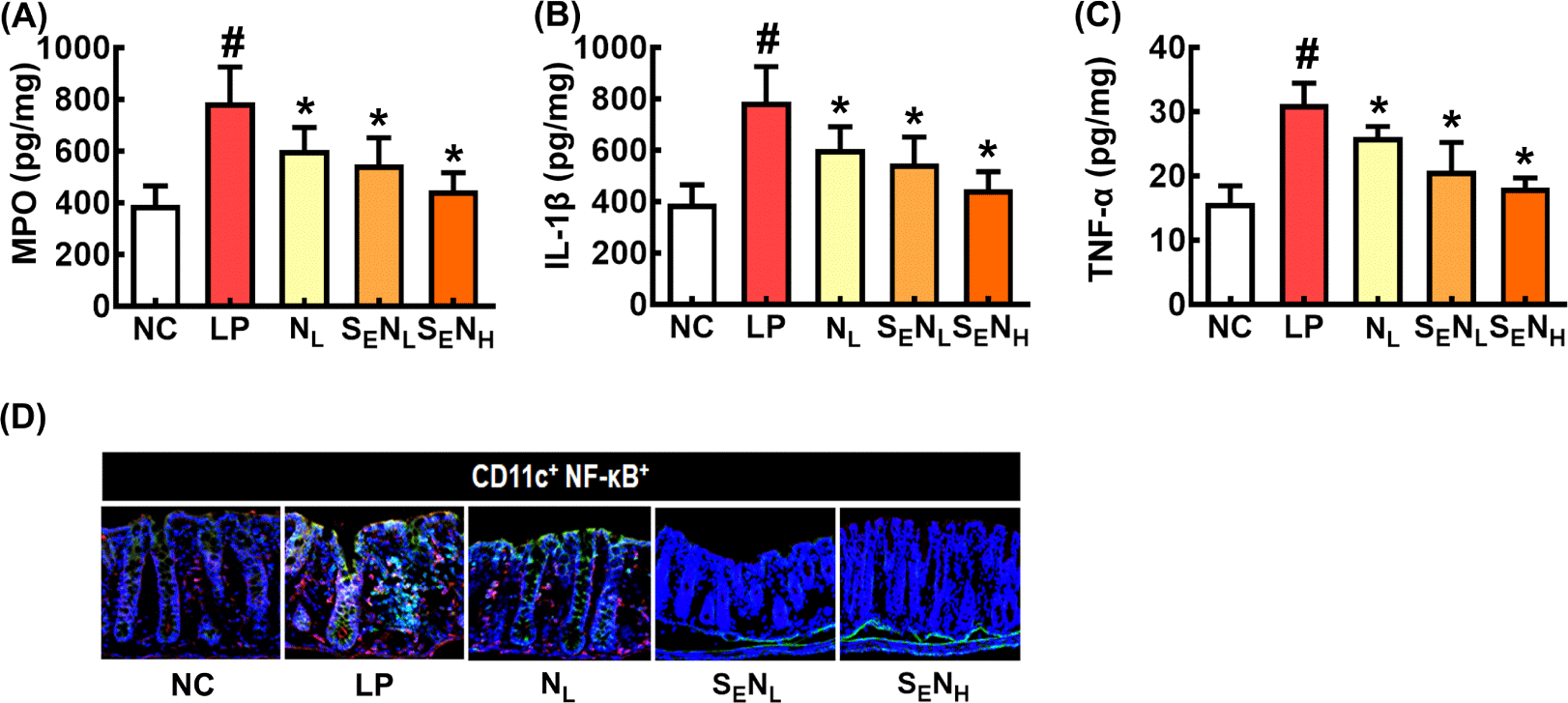
Oral administration of NK109 and heat-processed SE mix increased cognitive function in aged mice (Yun et al., 2023). Therefore, to investigate whether SE could increase the CI-ameliorating effect of NK109, we investigated the effects of NL, SENL, and SENH on LPS-induced CI in mice (Fig. 4). NL, SENL, and SENH all alleviated LPS-induced CI-like behaviors (Fig. 4A-C). They suppressed IL-1β and TNF-α expression and NF-κB+Iba1+ cell number in the brain, whereas BDNF expression and BDNF+NeuN+ cell number increased (Fig. 4D-H). They also decreased LPS-induced myeloperoxidase, IL-1β, and TNF-α expression and NF-κB+CD11c+ cell number in the colon (Fig. 5). Of these, SENH most potently alleviated CI-like behaviors, down-regulated proinflammatory cytokine expression in the brain and colon, and up-regulated BDNF expression in the hippocampus, followed by SENL and NL. However, there were not significant differences between them.
DISCUSSION
Brain and gut are at the bidirectional cross-talks through neural, immune, and endocrine networks (Cryan et al., 2019; Maqsood and Stone, 2016). Gut inflammation significantly increased the occurrence of dementia (Jang et al., 2018). The attenuation of gut inflammation by probiotics or drugs alleviates CI and depression (Park et al., 2020; Sakurai et al., 2022; Yang et al., 2020; Yun et al., 2021). Anti-inflammatory Limosilactobacillus mucosae NK41 mitigated LPS-impaired cognitive function and gut inflammation in mice (Lee et al., 2019). Lactiplantibacillus plantarum C29 and DW2009, a C29-fermented soybean, increased cognitive function and alleviated colitis in 5xFAD mice (Lee et al., 2018). They also alleviated CI and gut inflammation in mice treated with LPS or D-galactose (Lee et al., 2021a; Woo et al., 2014). Furthermore, DW2009 improved cognitive function in patients with mild CI (Hwang et al., 2019). These results suggest that anti-inflammatory probiotics may alleviate CI as well as gut inflammation in vivo through the activation of gut-brain axis.
In the present study, NK109 improved LPS-impaired CI, neuroinflammation, and colitis in mice. In particular, NK109 suppressed proinflammatory cytokine expression and NF-κB-positive immune cells in the colon and brain. In the previous study, NK109 attenuated Escherichia coli-induced CI and depression in mice by regulating NF-κB activation and gut microbiota dysbiosis and cognitive function in aged mice (Yun et al., 2020). These findings suggest that NK109 may attenuate CI by suppressing inflammatory response.
When NK109 was combined with SE, the combinations also alleviated LPS-impaired cognitive behaviors, suppressed NF-κB-positive cells and proinflammatory cytokine in the brain and colon, whereas BDNF level and BDNF-positive cells increased. Moreover, SENH more potently alleviated CI than SENL or NL. Although the efficacy of SENH against cognitive impairment was not significantly different to that of NK109 or SENL, the combination of NK109 and SE additively alleviated CI and colitis. In addition, NF-κB activation-induced neuroinflammation suppresses BDNF expression in the brain (Carreño et al., 2011; Kairisalo et al., 2009; Lee et al., 2021a; Marini et al., 2004; Ying et al., 2002). These results suggest that the combination of NK109 and SE may strongly alleviate CI by suppressing inflammatory responses and inducing BDNF expression. Moreover, the combination of probiotics with natural products may be beneficial for improving their efficacies.
In conclusion, heat-processing may increase the efficacy of SE on CI. The combined treatment of SE and NK109 may additively mitigate CI by the regulation of NF-κB- mediated BDNF expression.







